Introduction
Plasmas operating at atmospheric pressure in ambient air produce a wide range of reactive chemical species. When an atmospheric plasma is allowed to interact with water, a unique mixture of biochemically reactive chemistries occur, and the resultant chemically active water is referred to as Plasma Activated Water (PAW). Due to its unique biochemical activity, PAW is a promising technology in food, agricultural and biomedical industries and is a possible environmentally friendly alternative to chemical fertilisers. PAW has been shown to be effective at food decontamination, seed germination and improved plant growth [1-3].
Plasma-water chemistry
Atmospheric plasma generates reactive oxygen and nitrogen species (RONS) that interact with, and are incorporated into, water. The types and relative concentration of RONS are dependent on the method of plasma generation and on the specific gas used. Typical long-lived species can include nitrites (NO2-), nitrates (NO3-), ozone (O3) and hydrogen peroxide (H2O2). Short-lived species, such as hydroxyl radicals (·OH), superoxide (O2-), singlet oxygen (1O2), nitric oxide (NO·) and peroxynitrite (ONOO-), exist only for short periods with half-lifetimes of the order of a second.
The concentration of reactive species existing in the water yield a solution with higher oxidation-reduction potential (ORP), lower pH and higher conductivity than non-treated water, and have significant concentrations of acidic substances such as nitric (HNO3) and nitrous acid (HNO2). The synergistic effects of RONS in PAW is responsible for it’s biochemical properties and its many applications.
Figure 1 depicts the formation of RONS with a typical atmospheric plasma jet, within the plasma plume, gas region, gas-water interface and bulk water.
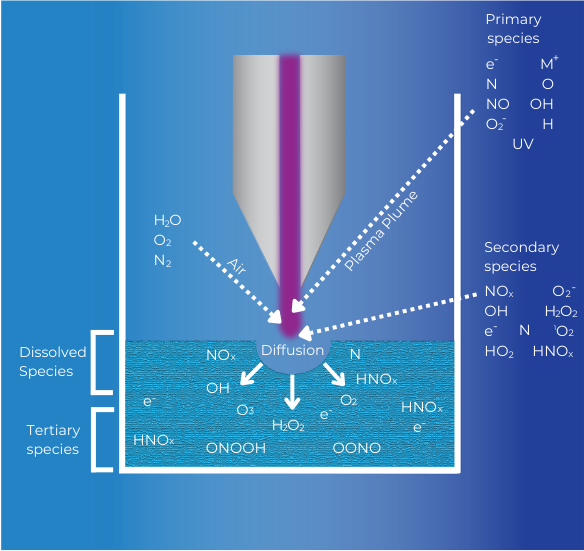
Figure 1. Schematic of the active species produced in PAW using an atmospheric plasma jet with process gas M.
Experimental
De-ionised (DI) water was exposed to the Cirrus plasma jet to produce PAW as shown in figure 2. The plasma jet had a constant power of 310 W and was situated 5 cm away from the surface of the water. The Cirrus produces a ~10 mm diameter jet of reactive plasma species using compressed air as the process gas. The standard Cirrus design and operating parameters produces oxygen and NOx radicals with only very low concentrations of ozone.
Figure 2. The experimental arrangement with mounted atmospheric plasma jet and beaker with 200 ml of DI water (left). Image of plasma plume interacting with the water (right).
DI water of volume 200 ml was treated for times ranging from 1 – 30 minutes. Immediately following treatment, measurements of pH and conductivity were performed using pH and conductivity probes. 30 ml of PAW was then transferred into glass vials for mass spectrometry measurements with the Hiden Analytical HPR-40 DSA membrane inlet mass spectrometer (MIMS), shown in figure 3.
Figure 3. The Hiden analytical HPR-40 DSA membrane inlet mass spectrometry system (left) and the pervaporation principle of the inlet probe (right).
The instrument settings used for PAW sample analysis were 70 eV electron energy and m/z survey scans from 0 – 100 amu. The untreated (background) DI water spectrum was subtracted from each treated sample spectrum to reveal the changes in its constituents.Results
Membrane Inlet Mass Spectrometry
An example survey mass spectrum for the 20 minute treated water sample case is shown in figure 4a, and the corresponding background subtracted spectrum in figure 4b.

Figure 4. Example mass spectra of plasma treated DI water (a). Example mass spectra of plasma treated DI water with background subtraction (b).
The background subtracted data revealed significant changes in the intensity of the m/z=30 (nitric oxide NO) and m/z=47 (nitrous acid, HNO2) peaks, which were then chosen for subsequent analyses.
Figure 5 shows the evolution of m/z = 30 and m/z 47, which increase for successive plasma treatment time until ~ 12 minutes when the concentration falls and plateaus at slightly lower concentrations. No other significant changes in detected species were observed.

Figure 5. Intensity of m/z = 30 and m/z = 47 for increasing treatment time.
pH and Conductivity
Figure 6 shows the measured pH (a) and conductivity (b) values. Treatment time 0 corresponds to untreated DI water. A significant drop in pH was found even after short treatment times. For example, untreated DI water had an initial pH of 6.96 and after 1 minute of plasma treatment, reduced to 3.85. At successively longer treatment times, the pH continues to drop but at a slower rate, settling to values close to 3.00. Conductivity on the other hand continues to increase significantly with treatment time, with a maximum of 529 µS/cm at 30 minutes of plasma treatment.

Figure 6. Measured pH (a) and conductivity (b) of the treated samples for successive treatment times.
Nitrate Test
The presence of nitrate and hydrogen peroxide was also measured using colour change test strips. It was found that all treatment times > 4 minutes registered a maximum colour change, indicating nitrate levels > 500 ppm. Therefore, separate experiments using shorter treatment times were performed to identify the incremental increase of nitrates in the solution.
Figure 7 shows the difference in nitrate levels for increasing plasma treatment times, up to a maximum of 3 minutes, and indicates an approximately linear increase over the treatment period.
It should be noted that there were no visible increases in H2O2 for the parameters explored here.

Figure 7. Nitrate test strips for increasing plasma on time (left to right)
Effect on Cut Flowers
Freshly cut identical roses were placed in vases with equal amounts of PAW (right vase) and untreated DI water (left vase). The roses were left undisturbed and were recorded over a 7 day period. Snapshots of time-lapse photography are shown in figure 8 below.

Figure 8. Effect of PAW on cut flowers over a 7 day period.
Discussion
Membrane inlet mass spectrometry was used to identify dissolved species. To our knowledge, this study is the first of its kind to use MIMS as a method of identifying dissolved gases in PAW. The intensity of nitric oxide (NO) and nitrous acid (HNO2), m/z = 30 and m/z = 47 respectively, increased to a plateau with plasma treatment time. The NO is likely to be formed from the ionisation of the parent HNO molecule in the mass spectrometer ion source however the ration of m/z(30):m/z(47) is higher than expected and further work should aim to determine whether this is due to differences in the relative permeation/enrichment rates of different RONS through the membrane sampling material.
Using simple test strips, we have shown a significant increase in nitrate levels, even at short treatment times.
The freshness and lifetime of cut flowers is an important factor for their marketability. Cut flowers often suffer from decay around the cut surface of the stem due to microorganisms and are damaged due to the inflow of air, resulting in less absorption of water and hence shorter vase life [3]. The PAW created in this work acts as an antimicrobial agent and the increased concentrations of nitrogen containing species also act as a natural fertiliser. These are thought to be the reasons for the positive effects shown in figure 8.
Conclusion
Using the Henniker Cirrus atmospheric plasma system, we have created plasma activated water (PAW) with different and controllable conductivities, pH levels and reactive oxygen and nitrogen species, the latter being measured with colour change test strips and by the analysis of dissolved gases via membrane inlet mass spectrometry.
Observations of the effects of the plasma activated water upon the longevity and quality of cut flowers shows that PAW can act as a fertiliser and is bactericidal.
PAW produced using the Cirrus has applications in:
- Plant nutrition
- Plant health
- Seed treatment
- As a pH controller
- As a disinfectant
Video Footage
Plasma Activated Water (PAW) with the Henniker Atmospheric Plasma
Plasma activated water is an emerging technology in food, agricultural & biomedical industries. In this short video we use Henniker's Cirrus plasma to make PAW. Witness its effectiveness through a captivating timelapse of cut roses.
References
[1] Thirumdas, Rohit, et al. "Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture." Trends in food science & technology 77 (2018): 21-31.
[2] Guo, Dingmeng, et al. "Plasma‐activated water production and its application in agriculture." Journal of the Science of Food and Agriculture 101.12 (2021): 4891-4899.
[3] Kwon, Song, and Ju Hyun Ryu. "Effects of Plasma Activated Water on the Postharvest Quality of ‘Siberia’Lily." Journal of People, Plants, and Environment 21.2 (2018): 93-101.
[4] https://plasmatreatment.co.uk/products-services/our-models/cirrus
[5] https://www.hidenanalytical.com/products/gas-analysis/hpr-40-dsa/





















