What is surface energy and why is it important?
In this ‘Let’s talk About…’ article we discuss the measurement of surface energy and ultimately, how plasma treatment can be used to alter surface energy in order to improve adhesion and bonding.
What is ‘Surface Energy’?
What is surface energy and why is it important? Molecules within the bulk of a material are surrounded in all directions by other molecules. To create a surface, some of these bonds must be broken. This requires energy. Some of this excess energy is ‘stored’ in the surface because the molecules are no longer surrounded on all sides by the same molecules as in the bulk; there are unsatisfied bonds.
Surface energy is defined as the excess energy at the surface of a material compared with the bulk material itself.
Now let’s consider what happens when a liquid comes into contact with a surface. Liquids possess the well-known property of surface tension. If the molecules of the liquid are attracted to each other more strongly than to the surface (i.e. have a higher surface tension) then the liquid won’t wet the surface very well, instead forming beads.
 |
 |
Fig 1: Water beading on a low surface energy material (left) and water spreading on a high surface energy material (right)
Conversely, if there is a larger attraction to the surface, then the liquid will spread out more. It follows that if a particular surface has a higher surface energy it will wet more easily and, since the ability to wet a surface is a simple definition of the adhesion characteristics of the surface, it will be easier to glue/print/paint or bond to that surface.
Surfaces that have predominately carbon-hydrogen (C-H) bonds tend to have low surface energies and so do not wet easily. Surfaces that have lots of oxygen-hydrogen bonds (O-H) have higher surface energies and therefore better adhesion characteristics. Polyethylene and polypropylene are examples of low energy surfaces, most metals have a higher intrinsic surface energy.
Why is Surface Energy Important?
The data shown below gives examples of the surface energy of several common polymers and also the surface tension of common liquids. The surface tension of the liquids is, in each case, greater than the surface energy of each polymer. This would result in poor wetting of the surface by the liquid and in turn poor adhesion.
 How Can Plasma Treatment Help?
How Can Plasma Treatment Help?
Plasma treatment aims to convert low energy surfaces to higher energy surfaces by activating the surface. Activation is the process by which polar molecules (molecules containing oxygen) from the plasma are bonded to the surface. The resulting increase in surface energy for the above examples is shown below. This increase means that common inks and adhesives will now form very strong bonds to the plasma-treated materials.
 How is Surface Energy Calculated?
How is Surface Energy Calculated?
There are several methods for calculating the surface energy of a solid and all rely on the measurement of the angle that is formed at the liquid/solid interface.
 The most basic expression describing the above phenomena is Young’s equation, which describes the balance of forces shown above and gives a method of calculating surface energy:-
The most basic expression describing the above phenomena is Young’s equation, which describes the balance of forces shown above and gives a method of calculating surface energy:-

Where γs is the surface energy of the solid, γsl is the interfacial tension between liquid and solid, γl is the surface tension of the liquid, and θ is the contact angle of the liquid on the solid. Commercial contact angle measuring devices employ various additional measurements and assumptions to determine the value of surface energy from the above equation combined with the measurement of contact angle.
Which other tests can be used to measure Surface Energy?
Water Break Test
A water break test is a very simple and quick qualitative test used to ascertain whether the surface energy of a treated material is greater than 72mN/m, the surface tension of de-ionised water. Water is simply brushed over the treated material (the object can also be dipped in water if possible) and if beads are formed on the surface then the surface energy is lower than 72mN/m. If the water spreads and wets the surface, then the surface energy is greater than 72mN/m.
Dyne Test Fluids
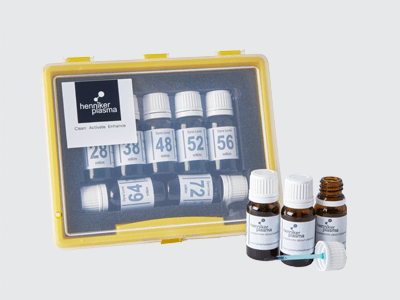
Dyne test fluids are an inexpensive, semi-quantitative test of surface energy which require no special training. Fluids are available in graduated energy level sets in the form of bottled ink with brush applicator or in the form of marker pens. In both methods increasing graduations (energy level) of the test fluid are simply drawn over the surface in turn. Low energy level fluids will bead up on the surface indicating that the surface energy is lower than that of the fluid used. When a particular fluid no longer forms beads but instead spreads evenly on the surface, the surface energy is approximately equal to that of the fluid. Surface energy is usually given in units of dynes/cm or mN/m and the test fluids are available in blue, red or green colour. Blue fluids are formamide based (toxic) and are formulated to DIN ISO8296 which means that the results are comparable between different manufacturing sites or laboratories. Red and green fluids are alcohol-based (non-toxic) and so strictly speaking not comparable between sites although usually absolutely fine for most instances.

Contact Angle Measurement
As mentioned earlier, contact angle instruments can optically analyse the shape of a drop of liquid in contact with a surface. This is achieved with a fast image capturing camera and analysis software. The drop shape and contact angle depend on both the topography and the surface energy of the solid surface. When attracted to the solid (material with higher surface energy), the liquid forms a drop with a low contact angle (θ < 90o). If repelled (material with low surface energy), the contact angle is high (θ > 90o). The contact provides a quantitative measure of surface energy.

The above image shows a typical contact angle image taken before and after plasma treatment. Measured surface energies are 56.8mN/m and 83.3mN/m
Adhesion Grid Cut Test
The grid cut test is a direct visual measure of the adhesion for thin films, i.e. paint. The paint film is scored in a cross-hatch pattern before an adhesive tape is applied and then removed. Poorly bonded material will be removed from the surface whereas permanently bonded material will remain on the surface. The method is covered under ISO 2409:2020.
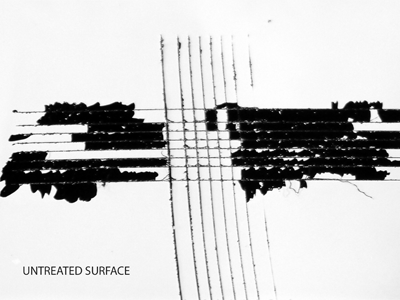
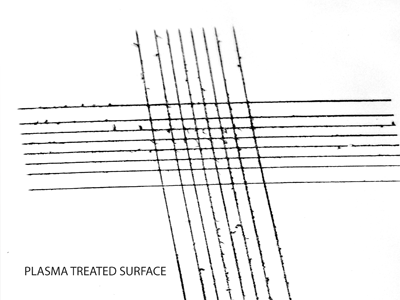
Henniker has extensive plasma processing and surface testing facilities to conduct surface energy testing and plasma process trials to determine the effectiveness of plasma treatment on a wide range of samples.



















