There are a number of quick and simple tests to determine the effectiveness of a particular plasma process upon a surface.
Adhesion and surface energy test methods have the benefit of being applicable to both untreated and treated surfaces and so also provide a useful comparison before and after processing. Each test is effectively providing information about the surface energy of the material being tested. Before describing the various test methods it is therefore useful to review what we mean by surface energy.
Surface Energy - Why it is so important to consider when using plasma treatment equipment?
Molecules within the body or bulk of a material are surrounded in all directions by other molecules. To create a surface some of these bonds must be broken, which requires energy. Some of this excess energy is ‘stored’ in the surface because the surface molecules are no longer surrounded on all sides by the same molecules as in the bulk; there are unsatisfied bonds on the surfaces.
Surface energy is defined as the excess energy at the surface of a material compared with the bulk material itself. Now let’s consider what happens when a liquid comes into contact with a surface. If the molecules of the liquid are attracted to each other more strongly than to the surface then the liquid won’t wet the surface very well, instead of forming beads. Conversely, if there is a larger attraction to the surface then the liquid will spread out more.
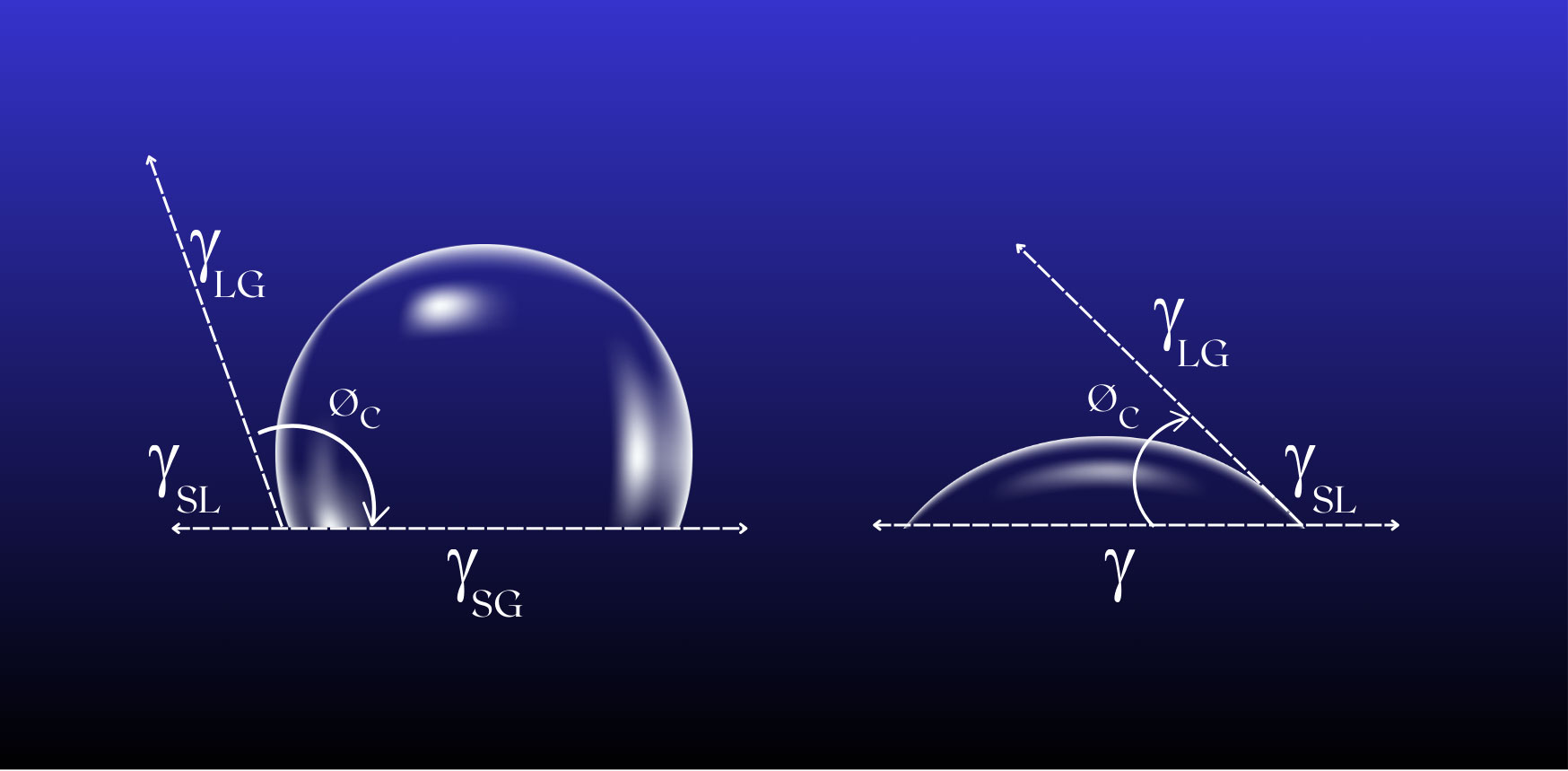
It follows that if a particular surface has a higher surface energy it will wet more easily and, since the ability to wet a surface is, in turn, a simple definition of the adhesion characteristics of the surface, it will be easier to glue/print/paint or bond to that surface.
Surfaces that have predominately carbon-hydrogen (C-H) bonds tend to have low surface energies and so do not wet easily e.g. wax. Surfaces that have lots of oxygen-hydrogen bonds (O-H) have higher surface energies and therefore better adhesion characteristics. Polyethylene and polypropylene are examples of low energy surfaces.
Plasma treatments aim to convert low energy surfaces to higher energy surfaces by removing hydrogen from the surface and attaching oxygen-containing species in its place. Other ‘functional groups’ can also be formed at the surface to give different and interesting properties.
Surface Energy and Adhesion
Adhesion and Surface Energy Explained.
The second in our series of videos about plasma treatment technology, this video explains what Surface Energy is and why it is so important to consider when using plasma treatment technology.
Surface energy test methods
We can go on here, to discuss the various methods available that best suit plasma technology and our customers.
Dyne Test Inks
Dyne test inks are a simple and inexpensive way to estimate surface energy and require no special training. They are available in graduated energy level sets in the form of bottled ink with brush applicator or in the form of marker pens.
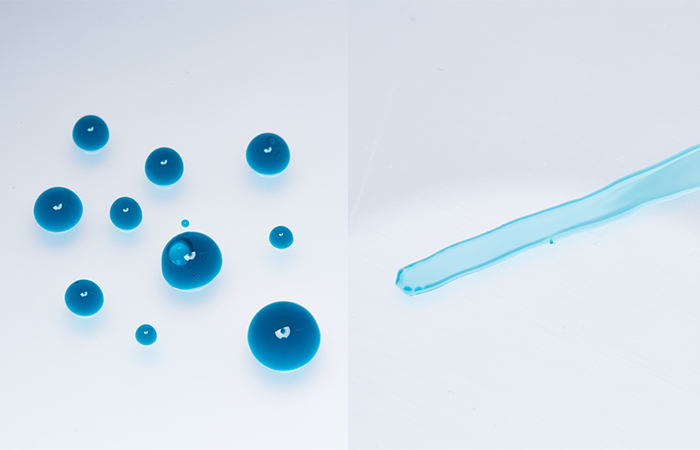
In both methods increasing graduations (energy level) of the dyne test ink are simply drawn over the surface in turn. Low dyne inks will bead up on the surface indicating that the surface energy is lower than that of the fluid used. When a particular dyne level no longer forms beads but instead spreads evenly on the surface, the surface energy is approximately equal to that of the dyne ink.
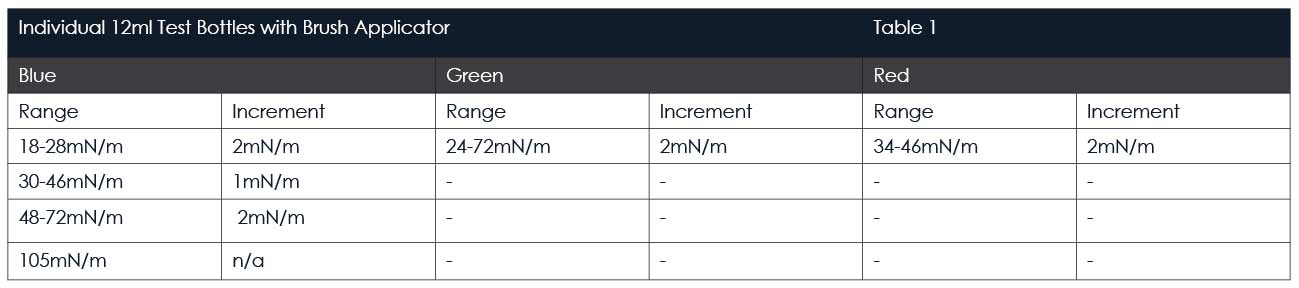
Surface energy is usually given in units of dynes/cm or mN/m and the dyne test inks are available in blue, red or green colour. Blue fluids are formamide based (toxic) and are formulated to DIN ISO8296 which means that the results are comparable between different manufacturing sites or laboratories. Red and green fluids are alcohol-based (non-toxic) and so strictly speaking not comparable between sites although usually absolutely fine for most instances.
Individual Bottles and Sets
Individual Dyne Test Fluid Bottles
Dyne Test Ink Bottles Sets
Available in sets containing six(blue) or seven (green and red) individual bottles in a sturdy case.
Our most popular sets are shown in the table below.

Click to view our full surface energy product range
Dyne Test Pens
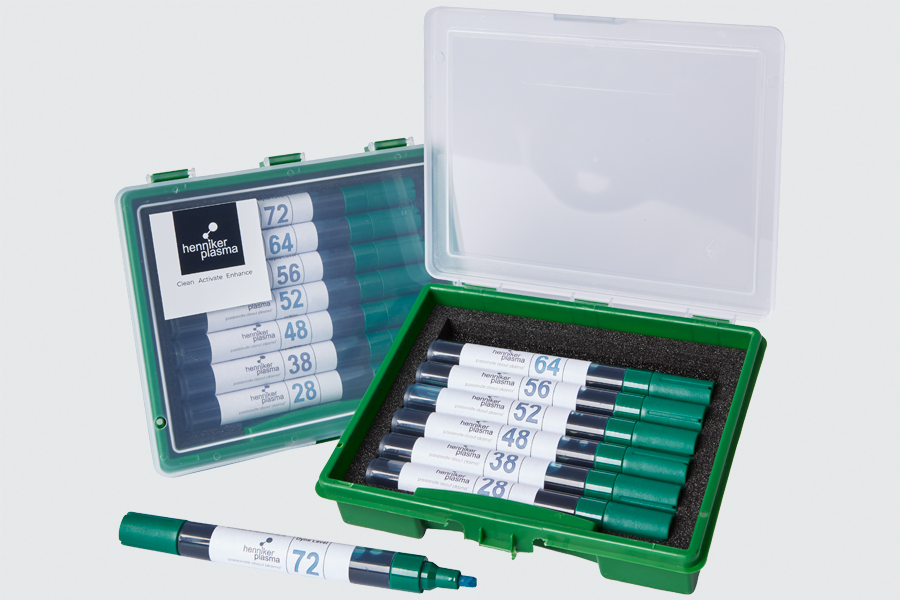
Easy-Test Pens
Easy-Test Pens are filled with 100ml fluid with any energy level from the green fluid range in table 1 above.
Quick-Test Pens
The Quick-Test pens are used as a quick quality test of whether or not a part has been plasma treated. They are refillable and come with a single ‘setting’
of 38 mN/m
Dyne Test Pen Sets
Henniker supply sets of Dyne test pens, available with graduated levels of surface tension. The Dyne pen is applied to the surface under study and simple visual observation of wettability is made. Using Dyne pens of increasing surface tension provides rapid identification of the approximate level of the surface energy of the sample,
Surface energy is normally measured in energy units called dynes/cm or mN/m. Our Surface Energy Test Pens Set contains individual pens covering the range 34 mN/m to 72 mN/m in 2mN/m increments.
Click to view our full surface energy product range
Test kit for silicone contamination
Silicones are polymers containing silicon, carbon and oxygen. Silicones can be present on many surfaces due to mould release agents or simply from leeching from ‘clean’ packaging, resulting in poor adhesion and bonding characteristics.
![]()
Unlike carbon-based polymers, however, only the organic functional groups can be removed by plasma treatment, leaving a non-volatile silicate surface.
A simple test method for the presence of silicone on a surface will, therefore, highlight potential issues in manufacturing steps or indeed in post-treatment packaging that may affect an otherwise pristine plasma-treated surface
Contamination Test Kit
The test kit contains everything required to test for silicone contamination together with simple to follow instructions. It is available in a small hard carry case and therefore suitable for ‘at-site’ testing.
Click to view our full surface energy product range
Colour change plasma indicator labels

Available in a range of sensitivity levels, our Plasma Indicator Labels are an indispensable tool for any operation where surface treatment integrity is paramount. They are easy to apply, require no additional equipment, and provide results within seconds, streamlining your quality assurance process and saving valuable time.
Each label is carefully formulated to react to specific surface energy thresholds, changing colour to signify whether the surface has achieved the desired energy level. This immediate visual feedback is crucial for maintaining consistent quality control during production runs and for validating surface preparation before further processing or coating applications.
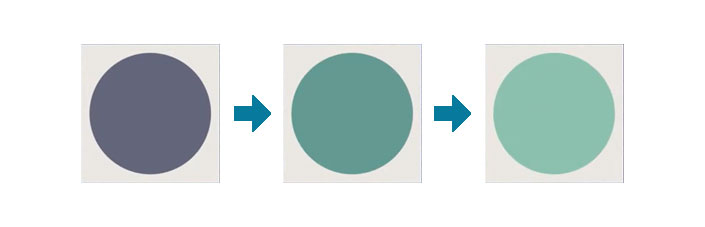
Plasma indicators from non-treated to effectively treated.
Click to view our full surface energy product range
Grid cut test
The crosshatch adhesion test is a simple and effective method for determining the coating adhesion on a wide range of materials. The adhesion tester is ideal for testing coatings on flat surfaces and is available with one of three different spacings;
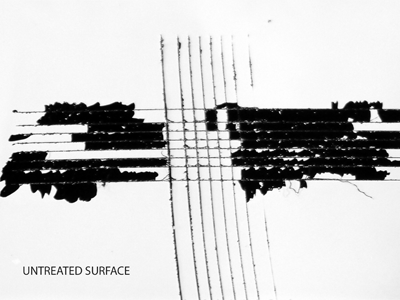 Untreated surface
Untreated surface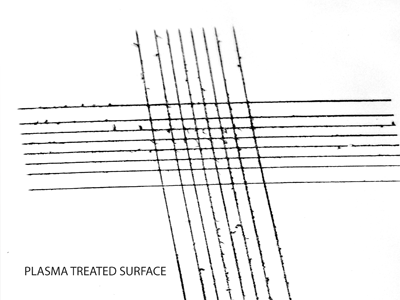 Plasma treated surface
Plasma treated surfaceGrid test cut methods (DIN EN ISO 2409) clearly demonstrate the enhanced bonding (right image) to plasma activated surfaces after treatment.
- 1mm spacing – for coating thickness <60µm (2.4mils)
- 2mm spacing – for coating thickness <125µm (5.0mils)
- 3mm spacing – for coating thickness <250µm (9.8mils)
Each cross hatch gauge can be supplied separately or combined in a kit with a standardised brush and x10 magnifier.
- Efficient cross hatch cutter with 8 cutting faces
- Anodised aluminium handle with a wheel for stable operation, ideal for test panels
- Supplied with an adjustment gauge for accurate positioning of the cutter face for better adhesion test results.
Click to view our full surface energy product range
Click here to download this Adhesion and Surface Energy Knowledge Article
Click here to to view our Knowledge Article 'Let's Talk About... Measuring Surface Energy'.




















